Safeguarding the Vaccine Cold Chain
With Sensire’s long experience in cold chain logistic solutions, real-time temperature and condition monitoring for vaccines becomes effortless.
Monitoring temperatures effectively in the pharma cold chain requires taking many things into consideration. Here are the basics to get you started.

Around a third of cold chain pharmaceuticals are put to risk by temperature excursion annually. Good practice regulations and guidelines have been created to correct this, but they allow for many different methods for managing temperatures in pharma logistics, some of which may not be very effective for their purpose.
From both patient safety and business perspectives, however, decreasing excursions would make good sense. The first step for this would be to have temperature monitoring that supports the specific logistics operation and delivers relevant data in order to optimize the operation. In the following article you can find out more about all of these things. You’ll also get an actionable list of steps for choosing the right kind of temperature monitoring system for your pharma logistics.We hope this will both inform and help you with your own operation! If you have questions or would like to know more about the solutions we offer for temperature monitoring in pharmaceutical logistics, please don’t hesitate to contact us!
Quick Links:
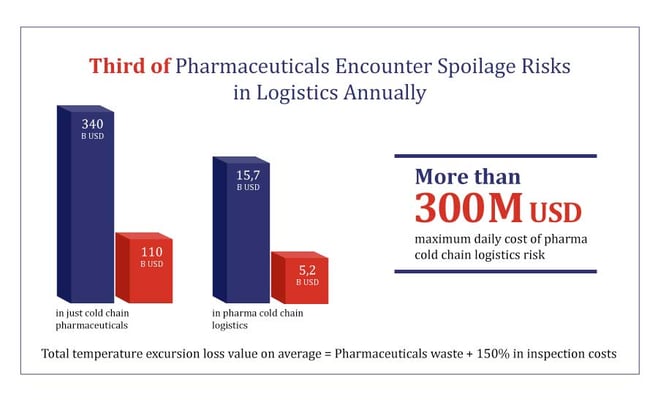
In a 76,4 billion USD global pharmaceutical logistics market, logistics decision makers report 63 % success with product damage and spoilage. That leaves a third of shipments that are presumably not successful. In a study about the vaccine cold chain, the results were similar:
“Among reviewed articles, the percentage of vaccine exposure to temperatures below recommended ranges during storage was 33% in wealthier countries and 37.1% in lower income countries. Vaccine exposure to temperatures below recommended ranges occurred during shipments in 38% of studies from higher income countries and 19.3% in lower income countries.”
If we consider even just the pharmaceuticals that require cold chain logistics, where the highest risk pertains to exceeded temperature limits, that means 110 billion USD worth of product at risk - 300 million USD daily! that’s a lot of pharmaceuticals potentially not reaching their recipients in time or at all.
It’s easy to see then how decreasing this amount would benefit pharmaceutical businesses a lot, even just by diminishing insurance payments.
Now, more and more of the highly valuable and temperature-susceptible biopharmaceuticals will be entering the market in the near future. This also means an individual temperature excursions’ price tag will be rising along with the rising product value. And naturally the total loss caused by an excursion is not just the worth of the pallet or box of product but also the costs of the excursion inspection.
Additionally, with pharmaceuticals we’re dealing with products that can mean life or death to someone. Any excursions therefore ultimately create waiting time, more costs and even possible physical harm and discomfort for the patient who should be receiving that medicine.
This means temperature and its successful maintenance will continue to grow in importance if we are to diminish the risk described above.
So, there are plenty of both business and patient safety reasons why it is vitally important to stop the excursions before they can affect product quality. To control these kinds of risks, the global pharmaceutical and regulatory community has put in place certain regulations, namely the GxP guidelines and regulations, which we’ll discuss more in the next section.
You can also read more about the challenges and solutions in the pharma cold chain in:
Because a patient cannot be allowed to receive degraded pharmaceuticals, various regulations are in place that tell the industry how to do manufacturing, distribution etc. according to best practices. These are known collectively as the GxP regulations.
The guiding principle behind all of these best practices is to make sure everything is done in such a way that the risk to the patient is minimized or completely eliminated. This includes but is not limited to: evolving processes that are right for the purpose; training all personnel who are in contact with the pharmaceuticals so that they are informed on their responsibilities and know how to exercise due diligence in their work; and creating a quality management system to monitor that the processes and people are performing according to standards.
However, complying with these regulations is not always as simple when you consider that different regions have slightly different regulations and they are also updated periodically.
For pharmaceutical logistics, the most important best practice is good distribution practice, which has been codified in various guidelines and regulations globally.

This means that keeping up to date with what is demanded from the different stakeholders in the pharma cold chain requires constant vigilance. And not only into what the regulation says but also the willingness and readiness to update practices to better ensure safety is maintained according to the guidelines.
And because evaluating the success of the processes and training is impossible without key performance indicators, you also need a way to monitor the success of your cold chain. One of the ways to achieve this is to implement sufficiently comprehensive temperature monitoring into the process, which we’ll explore more in the next section.
You can also read more about GxP in the following blog articles:
So, the simplest reason we need to monitor temperatures in pharmaceutical logistics is regulation – the various GxP guidelines and regulations demand specific data on pharmaceutical storage and transportation conditions in order to ensure the safety of the products.

However, while the regulations and guidelines set down what environmental factors need to be controlled and monitored, they make very little reference to how this should be accomplished. This has led to a number of methods being used concurrently in different pharmaceutical logistics operation, from manual checking of thermometers and indicators to continuously measuring sensors.
And as long as these methods can be shown to be effective in controlling the risk that environmental conditions cause to the pharmaceuticals, they will be in line with various good distribution practices.
However, the reasons for monitoring temperatures and other environmental factors shouldn’t be reduced to just following regulatory guidelines. This is because they’ve been created to ensure a standard level of pharmaceutical safety and quality is maintained. What they don’t really address are other considerations that may be as important to the storage operator such as efficiency, operating costs, and sustainability.
And to be clear, safety and high quality are paramount in pharmaceutical logistics. But the right kind of temperature monitoring system can help address both these regulatory concerns as well as those coming from the business side of things.
For example, an automatic temperature monitoring solution can both ensure quality and safety, as well as enhance efficiency by reducing manual work. By helping reduce waste and eliminate the need for shipping substitute products, it also helps with running a more environmentally sustainable business.
You can read more about how quality and efficiency can coexist in pharma cold chain logistics in this blog series on the subject:
So, there are many aspects to how and what kind of temperature monitoring to implement in the pharma cold chain. Choosing the right one depends on not only good practice regulation but also on many business factors that need to be considered in order to keep operations profitable.
Simply put, the more temperature exceptions the pharmaceuticals in your logistics process encounter, the worse the whole cold chain is doing (and vice versa), and that affects what kind of monitoring you want to implement.
Of course, as existing temperature measurement processes are part of your cold chain operations already, you need to also evaluate their current performance compared to what you want to achieve with your temperature monitoring. It may also be that your existing system is perfectly adequate for your needs, just not used to its full potential.
For starters, here is a handy chart of the most common temperature monitoring technologies and where and how they are used.
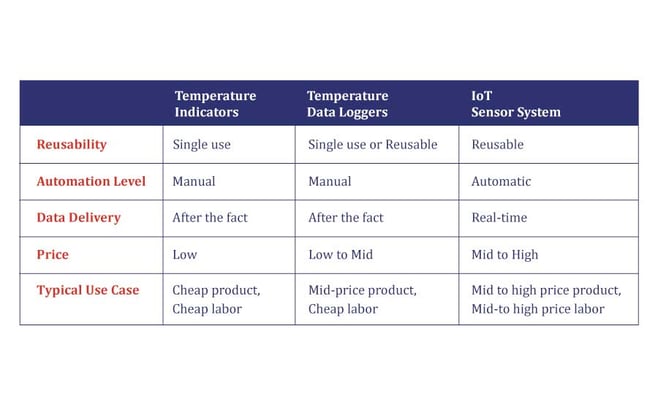
As you can see from the chart, both temperature indicators and data loggers demand manual work and deliver data only after the fact, so they are most commonly used where the product is less expensive and there is no lack of labor to handle it. They are also likely the most used methods for in-transit temperature monitoring.
The IoT system, on the other hand, is a handy tool for immediate deviation detection and handling. That’s why it is most often used with those more expensive pharmaceuticals and where either labor is more costly or the whole supply chain needs to be leaner, more environmentally friendly and more automated. This is also why it tends to cost more in the short term than basic indicators and loggers.
How thermostable the pharma product in question happens to be also affects directly what kind of solution is the minimum viable option to keep tabs on its temperature profile, which we’ll discuss next.
You can also read more about the evolution of temperature monitoring methods in the pharma cold chain in our article:
Now, just encountering an excursion along the supply chain does not automatically make a pharmaceutical go bad. The relative thermostability of various pharmaceuticals can actually handle a certain number of excursions, which is measured by mean kinetic temperature. This gives a sort of temperature budget to the pharmaceuticals, within which they maintain potency.
However, quite some money goes into inspecting the temperature exceptions and excursion in order to see whether their budget is within limits or if it has been exceeded.
That is because only those pharmaceuticals with an exceeded budget need to be quarantined and destroyed. The rest may still be used. However, whether this kind of budget inspection is possible or how long it will take (and cost!) depends significantly on the temperature monitoring solution that has been chosen.
For highly stable generics which are transported in a known environment without a great risk of unpredicted freak weather, for example, it may be practical to use temperature indicator strips for just making sure the medicines have remained within tolerable conditions. Once the temperature exceeds the safety limit, the indicator shows the product cannot be used anymore.
This would be an example of the low-price, highly stable pharmaceuticals where the loss is rare and cheap enough that the owner of the pharmaceuticals can take the loss of an even only potentially spoiled shipments.
For a one-of-a-kind gene-based medicine which you’ll be transporting halfway across the globe, however, you may want to have a system in place which’ll tell you right away if something threatens to go amiss. For this, an IoT-based, internet-connected monitoring system will work best.
Between these two extremes there are naturally any number of cases where it would be nearly impossible to choose the right temperature monitoring option without mapping the logistics process and discovering what are the needs and requirements caused by climate factors, regulations and a host of other such considerations.
However, it is not just the monitoring system that benefits from a thorough assay of your pharma cold chain – once the right option is chosen, the assay can also be used as a foundation to estimate risks, map the various lanes used for transportation, and other such activities that will ensure a safer cold chain for pharma products.
You can also ask for help in doing this. There are professional cold chain consults who will be delighted to help, but they will also cost significantly. Systems vendors are often also available to offer their expertise on their chosen subject matter. For example, you can take advantage of Sensire’s product managers and their know-how in pharma cold chain monitoring by booking a free consultation:
Of course, you can also do much of the work by yourself, and in the next section you can find the seven steps to choosing the right kind of temperature monitoring method for your cold chain operation.
So, if in most cases it is difficult to predict how well a certain temperature monitoring technology would fit your process and improve your performance, how do you go about choosing the one that’s right for you?
Well, here are seven steps that you can take to start looking for that Goldilocks (not too hot, not too cold, but just right) solution!
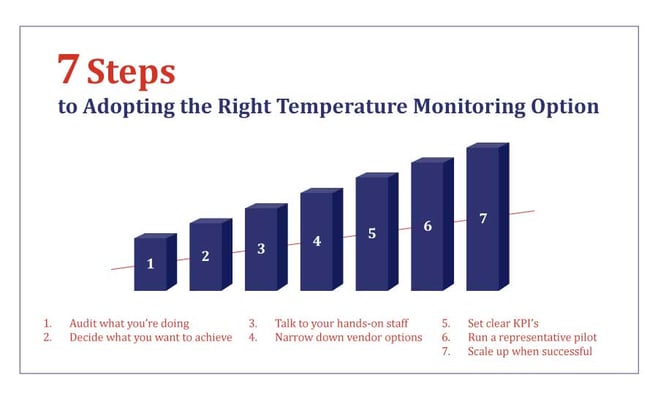
Often it may be difficult to recognize your need until something in your process breaks and you realize that it has to be fixed. What we’d suggest is to start with the numbers.
If you have sufficient data on your operation, it should be possible to discover if there are specific areas that constantly creates losses. For example, is there more waste in your pharma cold chain (or a part of it) than what you’d like there to be? Are your ETA’s all wrong? Do shipments get left in the wrong places and spoil as a result?
Or if you are missing some numbers altogether, that in itself is extremely telling of a blind spot in the cold chain.
Asking yourself these questions and verifying against numbers is the starting point to identifying the 20 percent of issues that generate 80 percent of problems.
If you’re handling pharmaceutical for someone else, you should also talk to your customers to make sure you know what are their current requirements and future expectations. You can then more easily offer services that are aligned with your customers’ wishes and deliver them the added value that differentiates you from your competition.
Once you list all the things that you want or need to improve, it’s time to move onto the next step.
Once you know how you are performing now, you should ask yourself how do you want to be performing? Is the primary need to show that everything is going fine, or are you looking at a situation where you need to improve your process to improve profitability and gain competitive advantage?
IF you can confidently say that you are doing fine and your temperature monitoring system is doing its job in proving this, congratulations! You’ve done your due diligence, have your temperature monitoring process in order and can continue doing what you’re doing.
IF that is not the case, however, you need to make sure you know what you are looking to improve before going forward on the list.
It may be preventing temperature exceptions more effectively, or reduce manual labor, or just gather data on compliance more easily. There may also be more than one objective you’re looking to reach.
Ultimately, however, you should know what you’re looking to address, because otherwise it’ll be very difficult to evaluate what kinds of action needs to be taken to achieve these improvements in youir pharma cold chain.
Once you know what needs to improved it’s time to look into how this can be done. The first thing we’d suggest is to talk to your team and hands-on staff. Ask them what they think is the biggest challenge in what you’re doing now and how would they solve it.
The people who are daily working with your cold chain are already experts on it and likely already have first-hand experience with some of or all of the same challenges you have identified in step one. By recognizing this you can potentially save yourself a lot of trouble.
In some cases, it may be that you aren’t even in need of a new temperature monitoring solution, but just need to tweak your existing one a little. It may be enough just to implement something as small as an adjustment to the standard operating process that everyone knows would help but haven’t thought to communicate to the people in charge of the SOPs.
However, even if you still need a new kind of temperature monitoring system after this, it’s good to get your team involved at an early stage. This way they can get used to the idea before you start running pilots at step six, or introduce full system upgrades at step seven, and you can also gather feedback at all stages of the process.
Now that you know what aspects of the process are not working to spec, it’s easier to start looking for the perfect monitoring solution that helps you correct them.
Here you may want to check back to that earlier table where we looked at different kinds of temperature monitoring systems in order to remind yourself where they are typically used and what you can expect them to deliver.
Of course, you want to make sure the solutions you consider can deliver the data they are supposed to, and in such a timeframe that best supports your operation.
However, selecting the correct temperature monitoring vendor cannot be reduced to just technology, and you’ll want to take in to account such other factors as level of service, price (of course), knowledge of field, references etc.
Here you may also refer back to your team, because they may have prior information on particular vendors and if their offerings have proven good or not. If you have a way of benchmarking what others in similar situations are using, it may be an easy way to narrow down the list of possible vendors.
Another thing you might want to keep in mind during this step are the 4 V’s of big data: velocity, veracity, volume, variety. This basically means that you should make sure you know what kind of data you need and how fast, and then see if the system you’re considering can provide it.
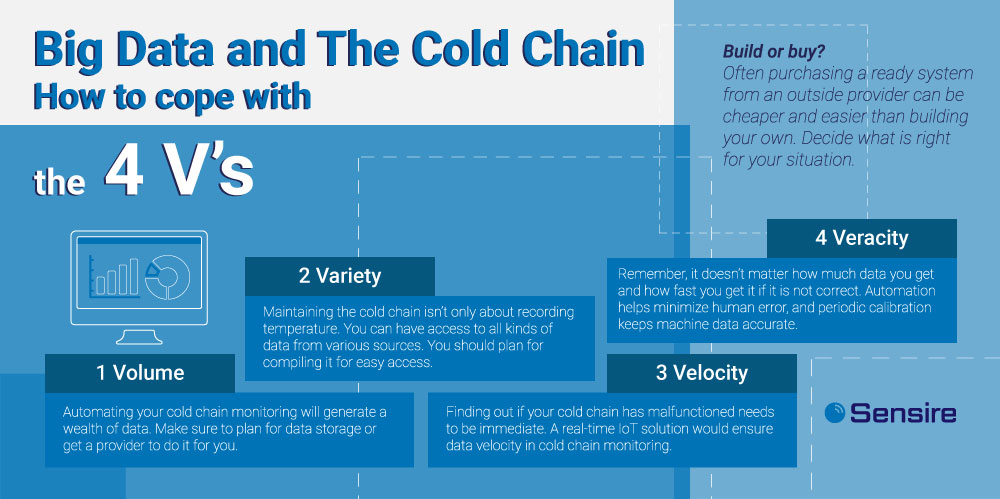
You’ll ultimately want to prune the list of potential system vendors until you can cut it down to maybe 2-3 options that seem likely. Of course, if you happen across one that is clearly the best option for you, you may want to contact them right away – but don’t throw away the rest of the list! You never know how someone handles their business regarding you until you have tested their service.
Now you have a few potential system vendors in mind, it’s time to make a plan on how to try them against each other. Traditionally this means running a pilot with the chosen system. However, before starting a pilot, you should come up with a few key KPIs that you can use to test the success of the pilot.
These may be some very specific things, like diminishing excursions by xx %, or some more broad ones like improving efficiency compared to the current way of doing things. Whatever they are, they should correlate with the goals you set in step 2.
Once you are satisfied that you KPIs reflect your requirements and can provide actionable results for evaluation, you can move on to the next step where you’ll find out how well the KPIs can be fulfilled by your set of potential systems.
Now we’re in the step where it gets interesting. You’ve chosen few vendors to pilot their systems and you know your KPI’s for evaluating success. It’s time to see how well will these systems perform in your actual pharma cold chain.
One thing you should consider in the pilot stage especially is that the situation should be as representative as possible, from setup to performance. This enables you to predict what kind of process to expect if you were to choose one and start scaling it up.
For example, you might consider that if you have to spend a lot of time on getting the system working in the pilot phase, would you be willing to go through the same thing in a much wider fashion in the scale-up phase. That may of course already be one of your KPIs for the pilot – what is the time from setup to undisturbed operation?
After the pilots are done, you can take some time to cross reference the results of the systems against each other. What is the easiest to use, what fixed the problem most efficiently, what was the one that gave the most comprehensive and clear data? Once you do the math from our KPIs, you should be able to see which one of the options has worked best for you.
Scale-up should be easy if you have taken the steps discussed above. You should know what you’re doing this for, how it should help and you have even tested that the system you are going with is delivering to your expectations and the set-up and use processes are already known to you.
Now you just need to do the last part at scale and start using it. Of course, suddenly having a lot more data than what you had before can be overwhelming at start. But if you have tested all facets of the system during pilot, you should have a good understanding of what kind of data you’re receiving and from where.
Then it’s just a matter of focusing on the most relevant matters in your pharma cold chain processes and starting to manage by exception. And by being able to concentrate on the challenges you won’t have to spend time worrying that something will pass you by and cause trouble further down the line.
Having procured the right temperature monitoring system for your specific operation, you should now be in an extremely good position to start eliminating previously existing challenges and improving further those parts of your pharma cold chain that were doing merely OK before.
Seven steps are not much, but as you can see, even those may take quite a lot of work. And what if you trip at the first hurdle? Are you sure you know how to recognize what you need, or that you know how to choose the right type of solution for addressing those needs?
One way to prevent this from becoming a problem is by taking advantage of outside help. There are many consultants available who are specializing in the field of pharma cold chains, although this can create significant costs.
There is also the option to try a free consultation from a vendor offering solutions. This will be much cheaper or free, but you run the risk of being aggressively sold to. However, this is a relatively uncomplicated way to get some pointers on what you can expect to find on the market. This in turn may help you when you go look at available systems for yourself.
We offer such service too, although we’ve decided that the initial consultation shouldn’t be about sales but rather about helping the customer. That’s why we’ve not given the responsibility for consulting to our sales, but to our product managers, who are primarily interested in how and if a product can fit a customer's individual pharma cold chain process.
And naturally we are also always happy to demonstrate our pharma cold chain specific digital temperature monitoring solutions for you!
With Sensire’s long experience in cold chain logistic solutions, real-time temperature and condition monitoring for vaccines becomes effortless.
Ensure the integrity and safety of temperature-sensitive pharmaceuticals with cold-chain monitoring and digitalization solutions.
Explore the critical shortcomings of manual temperature monitoring in pharmaceutical logistics and the devastating impact of such failures.
Stay up-to-date with Sensire's latest news. Enter your email to subscribe and get the latest insights and developments of our core product, new materials, blog posts and industry news.
We keep you informed!