EU guidelines on good practices in manufacturing (GMP) and distribution (GDP) keep updating with predictable frequency. Investigational medicinal products regulation will be coming once the Union manages to get their unified portal working (likely by the end of 2019 at the soonest), and while it does not specifically mention GDP, the EMA Q&A document mentions that the standards basically apply in storage and transport as well.
GMP Annex 1 is at draft stage and may go through considerable changes before being put to practice, so it’s anybody’s guess what eventually becomes of cleanroom guidelines. And EMA is also planning to revise the whole 1st chapter of GMP guidelines to demand a risk-based approach to drug shortages. This is almost universally expected to be the direction that the rest of GxP guidance will also increasingly take in the coming years. Many more changes loom on the horizon.
"Ensuring pharma quality will practically demand real-time monitoring”
Keeping up with the regulation and standards can be understandably daunting. The manufacturer/ wholesaler naturally must employ a qualified/responsible person to take care of compliance, but the workload of doing this may vary depending on what kind of methods are used by the wholesaler and their 3PL providers. 3rd party operators may experience less stress on compliance-related matters, delegating the final responsibility to the wholesale distributor, but they must also have methods in place to provide data on their compliance and audit trail segments. Not providing this risks a non-compliance report and possible cancellation of the original wholesaler’s distribution license, resulting in the 3PL operator losing business.
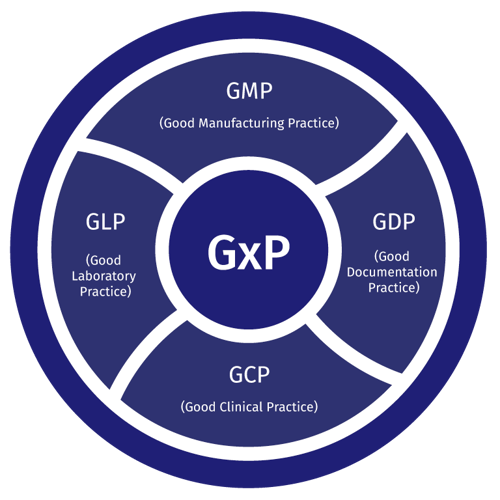
From GMP in manufacturing and GLP in laboratories to GDP in distribution, there is strict demand for temperature monitoring for the full pharma product lifecycle. Taking into account the fact that future GMP guidelines will most likely be focusing on risk assessment as the predominant way of ensuring pharma quality, the testing of conditions before starting and periodically during operations will practically demand real-time continuous monitoring in addition to risk spot checks. This may not be written into regulation yet, but there is very little doubt that this is what is implied. WHO for one already considers real-time monitoring the standard way of handling temperature tracking.
"Monitoring systems can vary wildly”
This is no small matter: as an expert in monitoring systems, we at Sensire can attest to the fact that real-time end-to-end monitoring really works in ensuring quality and minimizing waste. Therefore, this trend should be greeted with a warm welcome. However, not all systems are created equal.
Gathering data from disparate solutions and devices can turn out to be challenging when even the real-time monitoring systems can vary wildly. And some operations still cling to the 20th century pen-and-paper method of data recording. The wholesale distributor’s appointed responsible person must validate the information coming from all these and have access to an unbroken audit trail, so having to deal with data in multiple formats can create needless extra work. Of course, the data must also be immutable as required by GDocP in various GxP standards, whether it is gathered electronically or with pen and paper.
"A unified monitoring solution that complies with the spirit of GxP”
Clearly a unifying monitoring and data gathering system could help lessen this workload considerably, while also ensuring quality and compliance. When the solution is accurate enough from the start to fulfill the manufacturer requirements for pharma products monitoring, there will be no need to worry about complying with GxP regulations and standards. With correct installation and operating procedures, the system handles its own attached sensor network, and through integration it can gather data from other linked systems as well. This means an unbroken cold chain, or controlled room temperature, or whatever the customer’s need might be. Adopting a unified monitoring solution not only complies with the letter of the law, but also with its spirit.
Of course, this is not just a thought experiment: Sensire’s monitoring solution is just such a system, with extensive practical experience in various temperature and environmental conditions monitoring applications. Our objective for the Sensire logistics process & product quality management solution in particular is to make complying with GxP so easy that our customers only need to deal with the good working of their own operations, not with whether their monitoring solution is operational and functions correctly.
And for more information on this and other related subjects, why not check out our most comprehensive resource yet: Temperature Monitoring in the Pharmaceutical Cold Chain
Looking forward to more articles like this one? Follow Sensire on social media and you'll be the first to know when new articles turn up!